Introduction
In the pharmaceutical industry, water is not just a utility—it’s a critical raw material that must be manufactured and monitored with the highest level of precision and quality control. The increasing demand for safe, compliant, and efficient water systems has driven significant technological advancements. Among these, SCADA (Supervisory Control and Data Acquisition) and Remote Service Systems stand out as transformative solutions.
This article explores the integration of SCADA and Remote Service Systems in pharmaceutical water equipment, emphasizing their benefits, technical aspects, regulatory advantages, and real-world application. These systems ensure not only operational efficiency but also uncompromised product safety, compliance with Good Manufacturing Practices (GMP), and streamlined maintenance strategies.
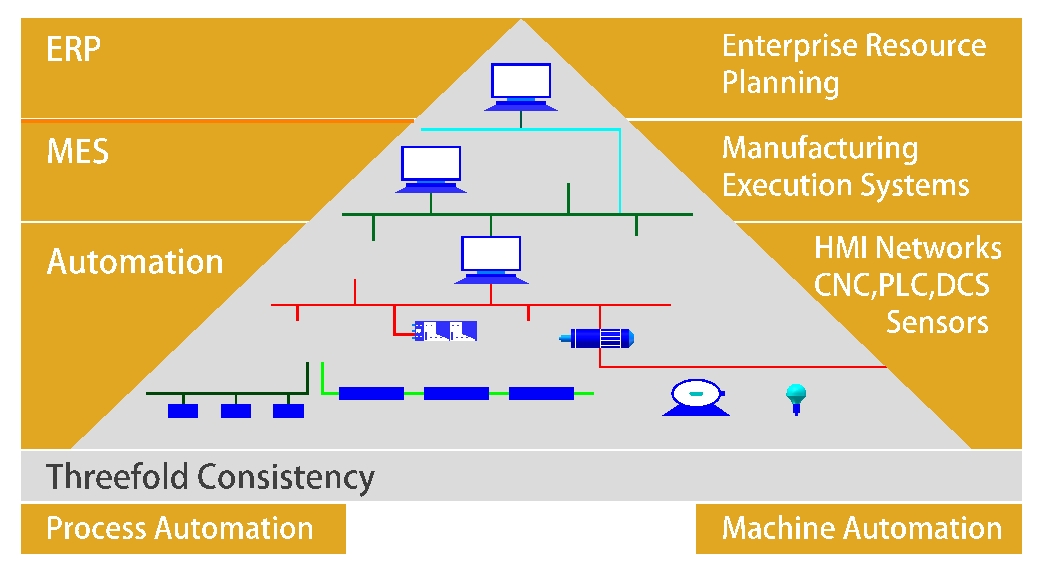
What Is SCADA?
SCADA is a system of software and hardware elements that allow industrial organizations to:
· Control processes locally or at remote locations
· Monitor, gather, and process real-time data
· Directly interact with devices such as sensors, pumps, and valves through human-machine interface (HMI) software
· Record events into a log file
In pharmaceutical water systems, SCADA serves as the digital backbone that monitors and manages processes like:
· Reverse Osmosis (RO)
· Electrodeionization (EDI)
· Ultrafiltration
· Storage and distribution of Purified Water (PW) and Water for Injection (WFI)
These processes require continuous surveillance to ensure compliance with pharmacopeial standards (e.g., USP, EP) and to maintain water quality integrity.
Core Functions of SCADA in Water Systems
1. Real-Time Monitoring
SCADA systems constantly monitor parameters such as conductivity, TOC (Total Organic Carbon), temperature, pressure, and flow. This data is visualized in real-time on HMI screens.
2. Alarm Management
When parameters exceed or fall below preset limits, SCADA triggers alarms and notifications. This ensures immediate response and reduces the risk of compromised water quality.
3. Historical Data Logging
SCADA securely logs all data for audit trails and long-term analysis, which is essential for GMP compliance and validation purposes.
4. Batch Reporting
In GMP environments, batch documentation is critical. SCADA systems automatically generate comprehensive reports that can be used for quality assurance and regulatory inspections.
Remote Service System: Extending the Power of SCADA
A Remote Service System integrates with SCADA to enable off-site monitoring, diagnostics, and maintenance. It allows authorized service teams to access the water system’s interface via secure VPN or cloud-based portals.
Key Features:
· Remote diagnostics of alarms and faults
· Firmware and software updates
· Predictive maintenance analytics
· Remote start/stop of processes (where permitted)
· Trend analysis and optimization suggestions
Regulatory Compliance and Cybersecurity
GAMP 5 and 21 CFR Part 11
Any SCADA system used in pharmaceutical manufacturing must comply with Good Automated Manufacturing Practice (GAMP) guidelines. Additionally, electronic records and signatures must adhere to FDA 21 CFR Part 11.
Audit Trails
SCADA ensures secure, time-stamped audit trails that track all system changes. These logs are tamper-proof and traceable to individual users.
User Access Control
Multi-level access control ensures that only authorized personnel can modify critical setpoints or configurations.
Cybersecurity in Remote Access
Remote systems must be built with end-to-end encryption, firewalls, and intrusion detection. Role-based access and two-factor authentication (2FA) are commonly deployed.
Applications and Use Cases
1. Water Purification System Monitoring
SCADA visualizes and controls the entire water purification chain, from pre-treatment to final loop distribution, ensuring real-time adherence to specifications.
2. Validation Support
During system qualification (IQ/OQ/PQ), SCADA provides valuable data for documentation. It can also assist in continuous process verification (CPV).
3. Troubleshooting via Remote Service
Example: A sudden drop in flowrate at the user point is detected. Service engineers log in remotely, review trends, pinpoint a fouled filter, and dispatch a technician with the correct part, reducing downtime from days to hours.
4. Cloud-Based Performance Dashboards
Some systems extend their remote services to cloud platforms, where managers can access high-level KPIs and OEE (Overall Equipment Effectiveness) metrics.
SCADA and Remote Service Systems have become indispensable in the operation of pharmaceutical water equipment. By offering real-time monitoring, robust data management, remote diagnostics, and predictive analytics, these systems enhance efficiency, reduce downtime, and ensure compliance with stringent industry standards.
As the pharmaceutical industry continues to embrace digital transformation, companies that invest in SCADA and remote service capabilities will be better positioned to meet quality demands, regulatory expectations, and market competitiveness.

